Postgraduate Seminar: Nikki Tzioumis
Wednesday, 4 November 2020. 11am – 12pm.
This seminar will be delivered via Zoom – Please email chemistry.researchsupport@sydney.edu.au for zoom link and password.
Nikki Tzioumis, PhD Candidate, School of Chemistry.
Host: Professor Kate Joliffe
Recognition of Sulfate via Squaramide H-Bond Donors in Aqueous Media
Sulfate is integral to many biological and chemical processes as well as a major contaminant in environmental and industrial systems.1 Therefore, developing a receptor for sulfate could have great potential use in numerous areas of study. As sulfate is found predominantly in aqueous media, a receptor should be soluble and functional in aqueous solutions. Development of neutral receptors can also be very advantageous as they can be used in a wider range of applications and pH ranges when compared to their charged counterparts.2 Due to the high solvation energy of sulfate in water (ΔG = -1090 kJ mol-1) binding to sulfate in aqueous systems is very challenging as a potential receptor needs to be able to overcome the strong sulfate-water interactions to be effective.3
From previous studies, it has been seen that peptide-based receptors containing the squaramide functional group exhibit strong affinity and selectivity for sulfate.4 However, these receptors also exhibited very low solubility in aqueous media. With the aim to develop a neutral water-soluble sulfate receptor, we synthesised a number of peptide-based squaramide receptors (Figure 1) where the squaramide side chain is appended with a hydrophilic moiety. During this study we investigated the effect the distance between the squaramide groups and the peptidic scaffolds had on the sulfate binding ability of the receptors, as well as the effects an additional squaramide moiety and increased preorganisation, in the form of macrocyclisation of the peptidic scaffold, had on this system. Binding affinities for these receptors to various anions were obtained via 1H NMR spectroscopic titration experiments in mixtures of water and deuterated dimethylsulfoxide (DMSO-d6) and binding to sulfate was observed in solutions containing up to 75% water in DMSO-d6.
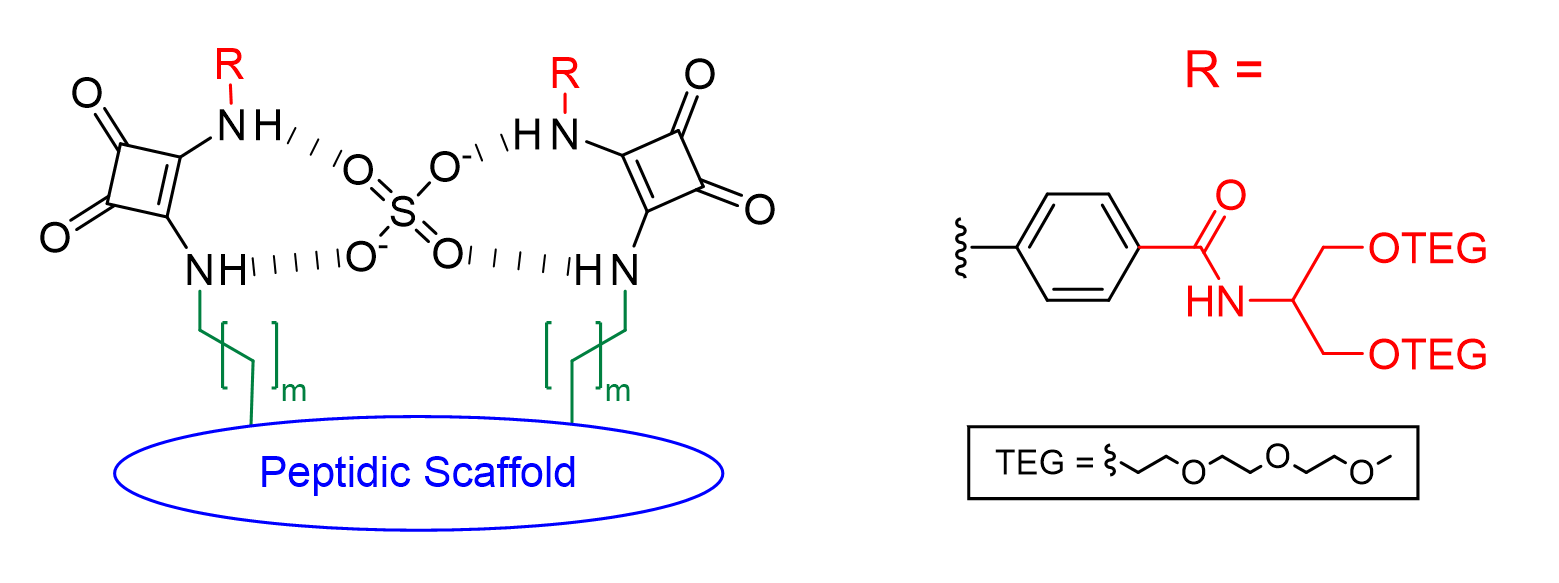
Figure 1. General Structure of peptidic sulfate receptors.
References:
(1) Baldwin, D. S.; Mitchell, A. Impact of sulfate pollution on anaerobic biogeochemical cycles in a wetland sediment. Water Res. 2012, 46, 965-974.
(2) Beer, P. D.; Gale, P. A. Anion Recognition and Sensing: The State of Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486-516.
(3) Kubik, S. Anion recognition in water. Chem. Soc. Rev. 2010, 39, 3648-3663.
(4) Elmes, R. B. P.; Yuen, K. K. Y.; Jolliffe, K. A. Sulfate-Selective Recognition by Using Neutral Dipeptide Anion Receptors in Aqueous Solution. Chem. Eur. J. 2014, 20, 7373-7380.

