RACI Athel Beckwith Lecture: Dr Lara Malins; Australian National University
Wednesday, 21 April 11:00am – 12:00pm
This seminar will be delivered via Zoom – Please email chemistry.researchsupport@sydney.edu.au for zoom link and password.
Speaker: Dr Lara Malins; Australian National University
Host: Professor Richard Payne
Title: Electrochemical Tools for Late-Stage Peptide Modifications
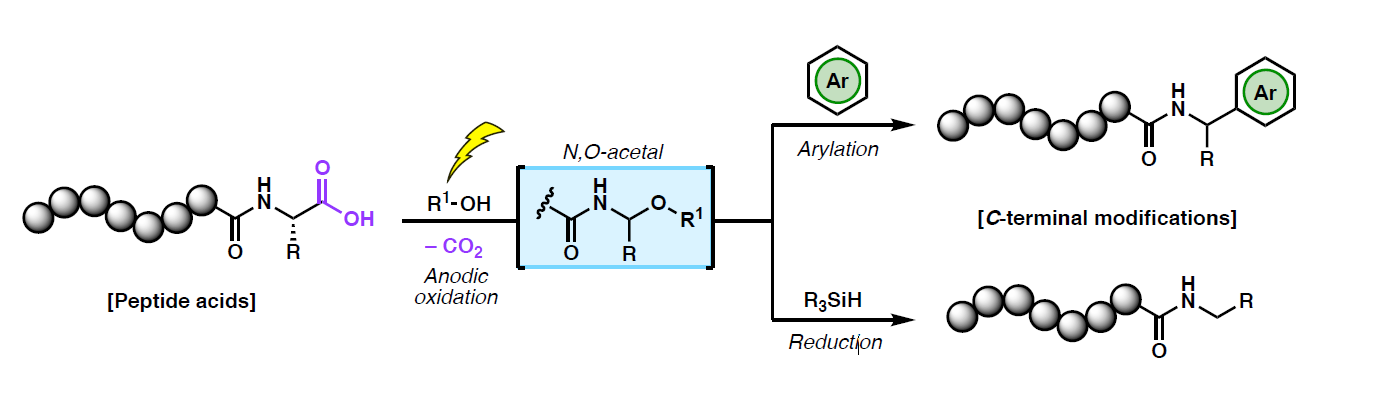
Abstract: The structural diversity of peptide natural products extends well beyond the incorporation of canonical amino acids. The precise enzymatic editing of ribosomally-produced peptide sequences is one of Nature’s most powerful strategies for structural and functional diversification.1 Consequently, synthetic strategies for the late-stage modification of peptides are in exceedingly high demand as tools for accessing and improving on Nature’s templates to
generate modified peptides as new therapies and functional materials. This talk will highlight our recent efforts toward electrochemically-enabled peptide
modifications. Despite the versatility and tunability of electroorganic chemistry,2 electrochemical transformations have been largely underexplored in the context of late-stage peptide modifications. Inspired by seminal work from the 1980s,3,4 we have leveraged the power of anodic oxidation to facilitate the divergent, decarboxylative C-terminal modification of unprotected peptides.5,6 This methodology is exemplified in the synthesis of bioactive
peptide natural products and related structural analogues. Given the compatibility of the method with conventional solid-phase peptide synthesis techniques, we envision that the electrochemical editing of peptide substrates will serve as a valuable addition to the toolbox of
late-stage modifications.
 References:
References:
1. Arnison, P. G. et al. Nat. Prod. Rep. 2013, 30, 108–160.
2. Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230–13319.
3. Renaud, P.; Seebach, D. Angew. Chem. Int. Ed. 1986, 25, 843–844.
4. Seebach, D.; Charczuk, R.; Gerber, C.; Renaud, P.; Berner, H.; Schneider, H. Helv. Chim.
Acta 1989, 72, 401–425.
5. Lin, Y.; Malins, L. R. Chem. Sci. 2020, 11, 10752–10758.
6. Lin, Y.; Malins, L. R., 2021, in revision.
Bio:

Lara Malins completed her B.A. in chemistry at Boston University in 2009 before relocating to The University of Sydney to undertake her PhD with Professor Richard Payne on the development of new peptide ligation strategies. In 2015, Lara joined the laboratory of Professor Phil Baran at The Scripps Research Institute as a National Institutes of Health postdoctoral research fellow. She returned to Australia in November 2017 to begin her independent academic career at the Australian National University, where her group focuses on the development of new synthetic methods for drug discovery, natural product synthesis, and chemical biology.

