School Seminar: Prof. George A. Koutsantonis; The University of Western Australia
Friday, 30 September 11:00am – 12:00pm
This seminar will be delivered in Chemistry Lecture Theatre 4 and Online Zoom Please email chemistry.researchsupport@sydney.edu.au for zoom link and password.
Speaker: Prof. George A. Koutsantonis; The University of Western Australia
Host: Dr Max Roemer
Title: Charge Transport Studies of Novel Photoswitches
Abstract: One of the primary issues associated with the practical aspects of using photoswitching molecules in molecular electronics is the significant geometrical change between the conducting and non-conducting states. This results in a flux in the interelectrode distance, restricting potential applications of such molecules outside of novel molecular junctions. An alternative to direct incorporation of the molecule in the junction is the incorporation of the switching functionality as a functionalised side group on a conjugated backbone. Spiropyrans exhibit fluctuations in geometry, corresponding to a difference of 2 Å when incorporated into a junction in a ‘side-on’ manner via the indoline N-atom and methyl groups on the same system.1 The end-on geometry is assumed to be more pronounced, leading to potential instability within the junctions.2 The established photoswitching of spiropyrans illuminate them as suitable candidates for the exploration of a functionalised side-chain approach. To this end, we previously explored ethynyl and ethynyl pyridyl functionalised spiropyrans.3,4 The stronger pyridyl binding groups made it possible to explore current-voltage properties of these junctions. Additionally, spiropyran junctions with strong binding Au-C contacts responded to mechanical stimulus. However, the introduction of the ethynyl moiety removed the light response of the spiropyran. Most recently, further evidence for switching spiropyrans under mechanical force in mechanically controlled break junctions has been provided.5 Inspired by the need to comprehensively study the conductance change of the isomerisation of spiropyran to merocyanine using light in single-molecule junctions, we have designed and synthesised several spiropyrans with suitable contact groups. We present here the study of two groups of linear-type spiropyrans and one group of t-shaped spiropyrans.
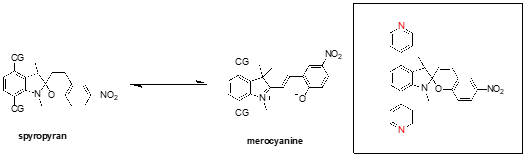
Reference:
- Sumit Kumar, Jochem T. van Herpt, Régis Y. N. Gengler, Ben L. Feringa, Petra Rudolf, and Ryan C. Chiechi Am. Chem. Soc., 2016, 138, 12519-12526
- Andrew C. Benniston, Anthony Harriman, Sarah L. Howell, Peiyi Li, and Donocadh P. Lydon, Org. Chem. 2007, 72, 888-897
- Mark C. Walkey, Chandramalika R. Peiris, Simone Ciampi, Albert C. Aragones, Ruth B. Domínguez-Espíndola, David Jago, Thea Pulbrook, Brian W. Skelton al. ACS Appl. Mater. Interfaces 2019, 11, 36886−36894.
- Mark C. Walkey, Lindsay T. Byrne, Matthew J. Piggott, Paul J. Low and George A. Koutsantonis, Dalton Trans., 2015, 44, 8812.
- Shuji Kobayashi, Satoshi Kaneko, Takashi Tamaki, Manabu Kiguchi, Kazuhito Tsukagoshi, Jun Terao, and Tomoaki Nishino ACS Omega2022, 7, 5578-5583.

