School Seminar: Prof. Julian Gale; Curtin University
Wednesday, 12 October 11:00am – 12:00pm
This seminar will be delivered in Chemistry Lecture Theatre 4 and Online Zoom Please email chemistry.researchsupport@sydney.edu.au for zoom link and password.
Speaker: Prof. Julian Gale; Curtin University
Host: A/Prof. Asaph Widmer-Cooper
Title: Getting Quantitative about Crystal Growth, One Step at a Time
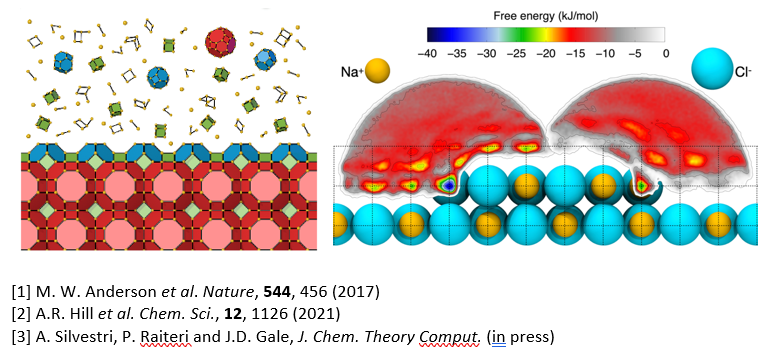
Abstract: Crystallisation is the fundamental process responsible for the creating of solid materials from species in solution, and as such plays a significant role in both the environment and industry. In the latter case, the ability to control crystal growth in order to manipulate the shape of the crystals formed is vital to the purification and active properties, such as drug release in the pharmaceutical sector. While the growth and dissolution of crystals has long been known to depend on the presence of surface features such as steps and kinks, there is often a lack of knowledge regarding the stability of individual sites, especially in the presence of a solvent. One recent approach to try to extract semi-quantitative thermodynamic data has been to use simulations within the CrystalGrower model [1,2] to reproduce experimental data from atomic force microscopy. However, this strategy currently requires hundreds or thousands of simulations to find the best match between the energies of surface sites and experiment.
In principle, techniques from computational chemistry are also capable of determining the thermodynamic stability of the surface sites that are critical to crystal growth. However, there are several challenges to overcome which mean that accurate free energies of surface sites in the presence of a solvent are extremely rare in the literature [3]. Here the free energies of kink sites for the seemingly simple case of rock salt (NaCl) will be determined using two different computational methods; one involving direct pulling of the ions away from the surface into aqueous solution and the other using alchemy to make the ions magically disappear and reappear in water. In what might seem like a challenge to the rule of Hess’s Law, the two pathways lead to similar, but not identical answers. Resolving this dilemma leads to insights into the consequences of the mineral-water interface, while determination of reliable free energies for a pair of kink sites is found to challenge expectations for NaCl.
Biography: Professor Julian Gale obtained his first degree from the University of Oxford in Natural Sciences (Chemistry), where he continued to study for a DPhil in the Department of Chemical Crystallography. After a postdoctoral position at the Royal Institution of Great Britain he moved to Imperial College London as a Royal Society University Research Fellow and subsequently Reader in Theoretical and Computational Chemistry. In 2003, he moved to his current location, Curtin University, as a Premier’s Research Fellow and now holds the position of John Curtin Distinguished Professor of Computational Chemistry. In 2018 he was awarded an Australian Research Council Laureate Fellowship and is a Fellow of the Australian Academy of Science. Research interests include the development and application of computational techniques to problems in areas including materials chemistry, crystallisation, geochemistry and mineralogy.

